C3 is focused on understanding all aspects of altered immune function in patients with CF. Altered immune function is responsible for many types of infection that damage the airways and causes the lung to fail. However, the development of new antibiotics is limited, treatment is toxic, and many organisms acquire resistance over time.
C3 Translational and Data Core was developed to provide clinically derived specimens to investigators studying immune dysfunction in CF. C3TDC has the ability to store a variety of specimens within the C3Biobank, as well as transfer fresh samples directly to investigators.
C3 Translational and Data Core was developed to provide clinically derived specimens to investigators studying immune dysfunction in CF. C3TDC has the ability to store a variety of specimens within the C3Biobank, as well as transfer fresh samples directly to investigators.
Purpose:
|
Specimen Request Process
|
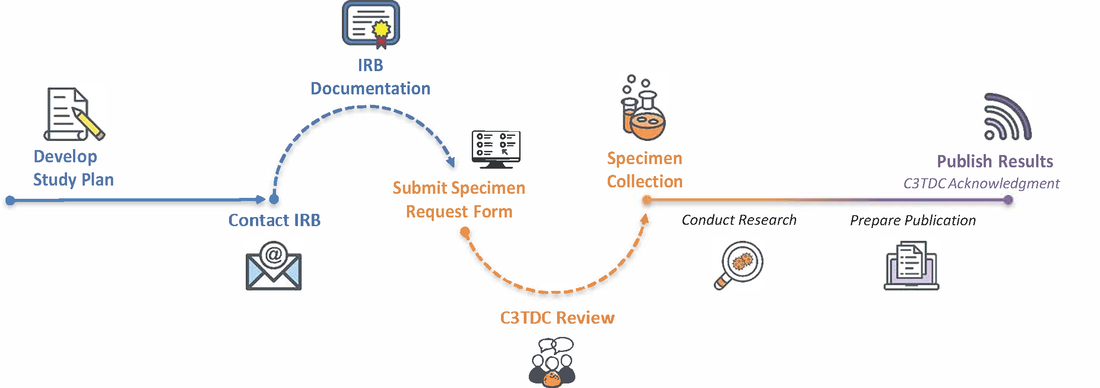
Step 1:
Develop Study Plan Write a Study Plan, which includes a full description of the proposed research study. Step 2:
Contact IRB Send an email to your institutional IRB. State that you are seeking a determination of exempt status for your study. Make sure to include a copy of your Study Plan. IRB Documentation Research may begin once you receive an email from the IRB stating your research is exempt. This email serves as your IRB Documentation. |
Step 3:
Submit Specimen Request Form Complete the online form to initiate the specimen request process. The Study Plan MUST be included with your submission. Requests can be submitted without IRB Documentation, but WILL NOT be approved until documentation is provided. C3TDC Review Approval will be granted based on feasibility (type, timeframe, and number of specimens required) and overall scientific merit as determined by the C3TDC Specimen Request Review Board (SRRB). Specimen Collection Once approved, C3TDC works with the primary contact, as noted on the Specimen Request Form, to arrange collection and/or delivery of specimens and data. |
Acknowledging C3TDC
Publish Results
Publications resulting from studies supported by C3TDC should include the following acknowledgement:
Publications resulting from studies supported by C3TDC should include the following acknowledgement:
This work was supported in part by the Cure CF Columbus Translational and Data Core (C3TDC). C3TDC is supported by the Division of Pediatric Pulmonary Medicine, the Biopathology Center Core, and the Data Collaboration Team at Nationwide Children’s Hospital. Grant support provided by The Ohio State University Center for Clinical and Translational Science (National Center for Advancing Translational Sciences, Grant UL1TR002733) and by the Cystic Fibrosis Foundation (Research Development Program, Grant MCCOY19RO).