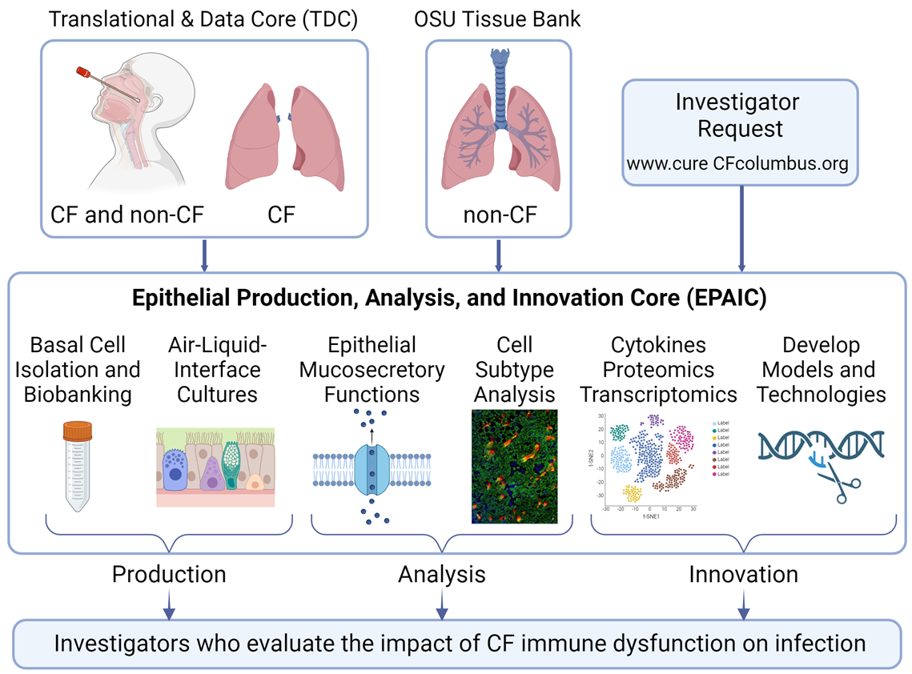
The C3 Epithelial Production, Analysis, and Innovation Core is supported by a Research Development Program Grant from the Cystic Fibrosis Foundation. The C3EPAIC produces primary human bronchial (HBE), bronchiolar, and nasal (HNE) epithelial basal cells from CF and non-CF donors and uses these regional stem cells to generate well differentiated air-liquid interface cultures. All cultures are from de-identified donors. The Core has a library of frozen HBE and HNE basal cells from CF donors with various CF genotypes and from non-CF donors. The EPAIC also prepares mouse tracheal epithelial cultures. The EPAIC conducts quantitative analysis of CFTR channel activity and expression, changes in the epithelial barrier and mucociliary clearance, and multiplex analysis of cytokine and chemokine expression/secretion. The EPAIC develops innovative methods that will facilitate the development of new treatments and curative therapies. Finally, the EPAIC also provides sample preparation and consulting related to ‘omics’ analysis including bulk and single cell RNAseq, proteomics, and gene editing in primary basal cells.
How to Obtain Cultures
Investigators who wish to use HBE or HNE cultures for their experiments may submit a brief description of their project to the EPAIC Co-I, Mark Peeples. The request will be reviewed for feasibility and scientific merit.
|
Once your project is approved, request HBE and HNE cultures by contacting Phylip Chen:
[email protected] |
Donor characteristics are available and may include:
- Age
- Sex
- CF transmembrane conductance regulator (CFTR) genotype
- Microbiology report
- Body mass index (BMI)
- Smoking history
- Pancreatic treatment
- CF-related diabetes (CFRD)
- CFTR modulators, corrector, and/or potentiator treatments [Kalydeco (ivacaftor); Orkambi (ivacaftor/lumacaftor); Symdeko (ivacaftor/tezacaftor); Trikafta (ivacaftor/tezacaftor/elexacaftor)]
Certified non-heterozygotic control cultures are available to CF investigators, from nine donors who do not carry any of the 99 most common disabling mutations in either of their CFTR genes.
EPAIC Organization and Workflow
Consulting
The C3EPAIC provides advice on experimental design.
Your Role
The C3EPAIC establishes ALI cultures on collagen-coated Transwells and feeds them for a week as they form tight junctions. Once the investigator is trained, they are provided with the cultures and enough freshly prepared medium to fully differentiate and maintain the cells during the experiment. If possible, we ask investigator to replace the Transwells used for their cultures to help defray the costs.
Analysis of Cultures
The C3EPAIC offers collaborative expertise in:
- RNA isolation for RNAseq and PCR arrays
- Mucus and mucin production (histology and immunofluorescence)
- Quantification of apically and basally released cytokines and chemokines
- Post-experiment culture staining to quantify the differentiated cell types in the HBE and HNE cultures (including ciliated, goblet, basal, and ionocyte cells)
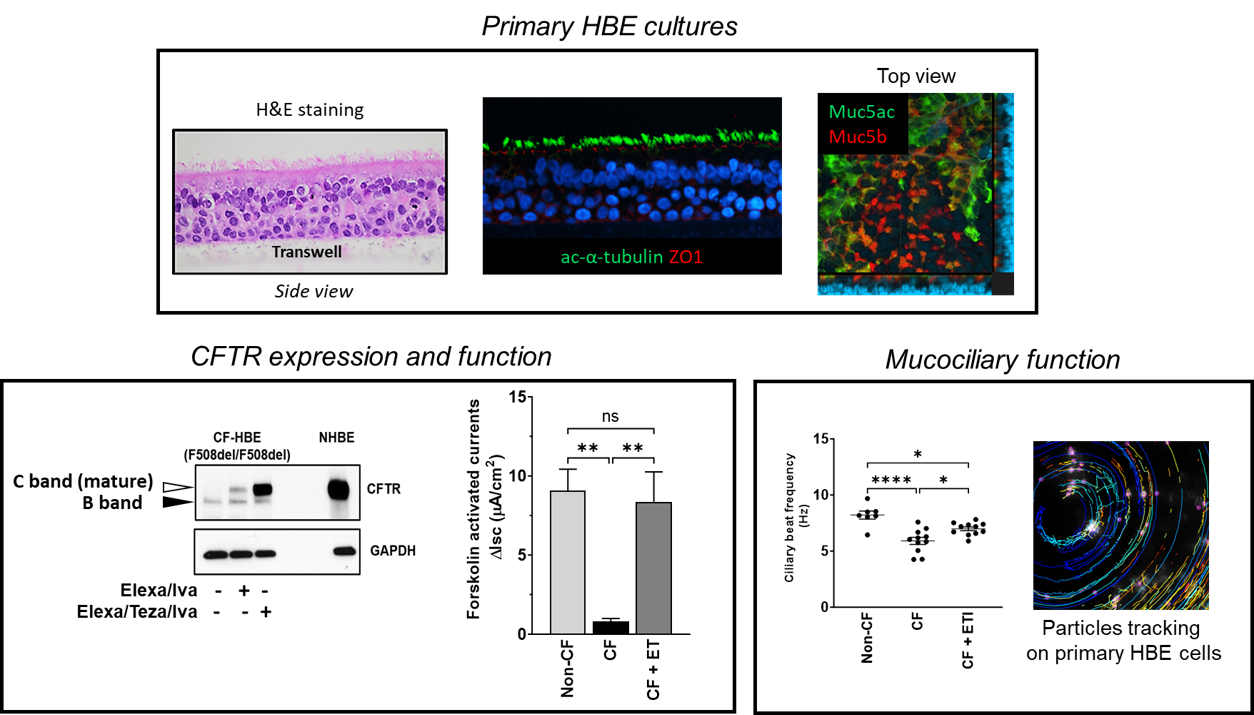
- Electrophysiology to determine CFTR function
- Immunoblotting to determine CFTR maturity
- Mucociliary function (airway surface liquid and ciliary beat frequency)
How are HBE cultures established?
Bronchial progenitor cells are extracted from the airways of explanted CF lungs or non-CF donor lungs by protease digestion. Nasal progenitor cells are isolated from nasal brushings of live donors. Basal cells are enriched, amplified, and biobanked for future use. Upon thawing, cells are plated on collagen-coated Transwell filters with medium in the upper and lower chambers, and grown in the presence of ROCK inhibitor for one week to allow tight junctions to form. The apical medium is removed, and differentiation medium is placed in the basal chamber. Over the course of the next three weeks, these cultures become pseudostratified, and differentiate into cells with beating cilia, goblet cells that produce mucus, and other cell types. In cultures from CF donors, cilia move the mucus around the well usually in a hurricane-like motion from the central point of the well.
Acknowledging C3EPAIC
Publications resulting from studies supported by C3EPAIC should include the following acknowledgement:
The Cure Cystic Fibrosis Columbus (C3) Epithelial Production, Analysis, and Innovation Core (EPAIC) at Nationwide Children’s Hospital (NCH) and The Ohio State University (OSU) provided primary human bronchial (or nasal) epithelial cultures, advice, and tools for this work, with the help of the NCH Biopathology Center Core and Data Collaboration Team. C3 is supported by a Cystic Fibrosis Foundation Research Development Program Grant (MCCOY17R2), the NCH Division of Pediatric Pulmonary Medicine (MCCOY19Ro), and the OSU Center for Clinical and Translational Science (UL1TR002733). Source tissues were provided by the Comprehensive Transplant Center Human Tissue Biorepository of The OSU Wexner Medical Center. (If nasal: by nasal brushings from volunteers.)
Testimonials:
|
The EPAIC “is also committed to develop new technologies (such as CRISPR knockout host factors); and the EPAIC Directors have a deep knowledge how to make and maintain the cultures.” JL
|
“EPAIC is an incredible source of primary bronchial epithelial cells for our lab. In addition, we have relied on them for performing Ussing chamber assays to measure CFTR function in CFTR gene corrected cells. They have been very timely in their response to our requests for cells and electrophysiological measurements. They are an incredible resource for our lab’s CF research.” SV
|
“I have received the most professional support from a Core that I have ever experienced. When I first developed this model for evaluating M. abscessus infection, the directors were all very responsive and facilitated interpretation of data. More recently data interpretation and suggested experiments led to funding from the CFF and a publication in preparation.” LHS
|